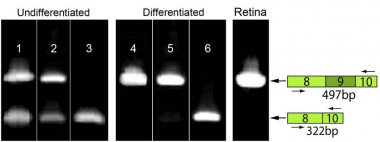
Development of treatments for Mendelian diseases will often require identification of disease-causing mutations and elucidation of the effect of these mutations on protein function. Wynn Institute scientists combined two relatively new technologies, exome sequencing and patient-derived induced pluripotent stem cells (iPSCs), to identify a new retinitis pigmentosa gene and demonstrate the pathogenic effect of its most common mutation. One important lesson that we learned from this study is that the blocking reagents used to remove repetitive DNA from sequencing libraries can also remove the genomic fragments bearing the mutation if the mutation is an insertion of a repetitive element as it was in this case. This finding has helped us tune our analytical tools to be more sensitive to insertions of this kind. However, the greatest significance of this paper is the use of patient-derived iPSCs to investigate the mechanism of disease. The inability to obtain samples of retinal tissue from living patients has frequently made it difficult to prove that a given variant observed in genomic DNA truly alters the function of that tissue. In this case, we were able to make patient-derived pluripotent stem cells from adult skin cells obtained from the proband and differentiate them into retinal progenitor cells. Using these cells, we discovered that MAK transcripts are differentially spliced during development and that undifferentiated cells lack exon 9 (the site of the mutation). We also discovered a previously unrecognized retina specific exon (exon 12) in humans whose inclusion in the MAK transcript does not occur in the presence of the exon 9 mutation. This improperly spliced transcript causes complete loss of the mature retinal protein. The same retinal progenitor cells used to demonstrate the pathogenic mechanism are currently being used to test the cellular efficacy of a lentiviral vector bearing a normal MAK gene.
This work was published in 2011 in Proceedings of the National Academy of Sciences 08:E569-76
