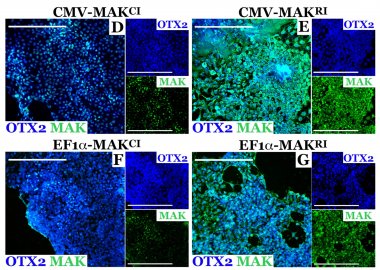
A team of IVR researchers characterized the safety and efficacy of a new treatment for early stage MAK-associated retinitis pigmentosa (RP). This research was recently published in the journal Gene Therapy (LINK).
This study evaluated patient iPSC-derived photoreceptor precursor cells treated with viral vectors containing either with the canonical or retina-specific isoforms of MAK. The results show that expression of the retina-specific isoform is needed for pronounced expression of MAK throughout the cell body. Defects in the MAK gene are also known to result in aberrant regulation of primary cilia length. To evaluate the effect of the supplementing MAK expression on primary cilia length, both patient-derived fibroblast cells and a zebrafish knockdown of mak were treated with the viral vector containing the retina-specific isoform. In both cases, expression of the retinal MAK isoform restored the ability to regulate primary cilia length.
These findings will help pave the way for initiation of a phase 1 clinical trial for the treatment of patients with MAK-associated RP.
