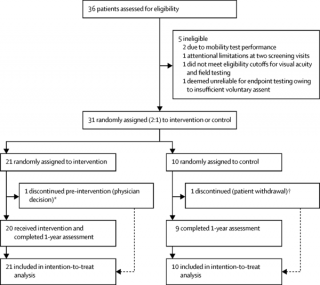
The RPE65 gene therapy study group at the Wynn Institute for Vision Research at the University of Iowa and The Center for Molecular and Children’s Hospital of Philadelphia published a paper in the journal, The Lancet, which showed the safety and effectiveness of voretigene naparvovec (the "new" name of AAV-RPE65 ) in a phase 3 FDA trial. This is an important milestone for gene therapy. As Dr. Stone recently published in the journal Ophthalmology, most inherited retinal degeneration genes will fit into the adeno-associated viral vector tested in this study. Patients that have other retinal genetic diseases may benefit from the results of this study.
The complete article is available at http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)31868-8/fulltext
